Di Sodium Edetate
Disodium Edetate Hydrate
Description
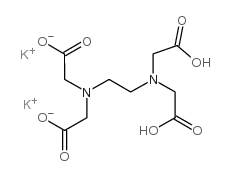
Disodium Edetate also known as edta disodium salt or disodium edta is a
chelating agent used in food, cosmetics, pharmaceuticals, and medicine to bind
and remove metal ions like calcium, magnesium, iron, and lead. A synthetic
compound derived from ethylenediaminetetraacetic acid (edta).
chelating agent used in food, cosmetics, pharmaceuticals, and medicine to bind
and remove metal ions like calcium, magnesium, iron, and lead. A synthetic
compound derived from ethylenediaminetetraacetic acid (edta).
Applications and Effect
Medical and pharmaceutical -treats heavy metal poisoning (e.g., lead poisoning), used in eye drops and injections as a stabilizer or preservative, helps prevent blood clotting in lab tests (by binding calcium)
Specifications
USP
BP
IP
JP
KP
USP

| Tests | Specifications |
|---|---|
| Description | Colorless crystals or white, crystalline powder. |
| Solubility | Freely soluble in water and very soluble in boiling water. Insoluble in alcohol. |
| Identification | A) solution (1 in 20) responds to the test for Sodium and for citrate. Sodium: A dense precipitate is formed. Citrate: A light red colour is produced. |
| Alkalinity | No pink colour is produced by 1 drop of phenolphthalein TS. |
| Water | Between 10.0% and 13.0%. Dry it at 180°C for 18 hours. |
| Alkalinity | Not more than 0.2 ml of 0.1M hydrochloric acid or 0.1M sodium Hydroxide is required to change the colour of the indicator. |
| Tartrate | No crystalline precipitate is formed. |
| Heavy Metals | Not more than 0.001%. |
| Assay | 99.0 % to 100.5 % (On Anhydrous Basis) |
BP

| Tests | Specifications |
|---|---|
| Appearance | White or almost white, crystalline powder or white or almost white, granular crystals, slightly deliquescent in moist air. |
| Solubility | Freely soluble in water, practically insoluble in ethanol (96 %). |
| Identification A. Citrates B. Sodium | 1. Violet colour, turning to violet-blue is produced. 2. A white precipitate soluble in 6M acetic acid. |
| Identification B. Sodium | 1. A dense, white precipitate is formed. |
| Appearance of Solution | Solution S is clear and colourless. |
| Acidity or Alkalinity | Not more than 0.2 ml of 0.1M hydrochloric acid or 0.1M sodium Hydroxide is required to change the colour of the indicator. |
| Readily Carbonisable Substances | The solution is not more intensely coloured than reference solution Y2 or GY2. |
| Chloride | Not more than 50 ppm |
| Oxalate | Not more than 300 ppm (calculated as anhydrous oxalic acid). |
| Sulphate | Not more than 150 ppm |
| Heavy Metals | Not more than 10 ppm |
| Water | 11.0 per cent to 13.0 per cent, determined on 0.300 g. |
| Assay | 99.0 % to 101.0 % (On Anhydrous Basis) |
IP

| Tests | Specifications |
|---|---|
| Description | White, granular crystals or white crystalline powder; odourless; Slightly deliquescent in moist air. |
| Solubility | Freely soluble in water; practically insoluble in ethanol (95%) and in ether. |
| Identification A. Citrates | 1. A dense, white precipitate is formed. 2. A yellow, crystalline precipitate is formed. |
| Identification B. Sodium | 1. A white precipitate soluble in 6M acetic acid. |
| Appearance of Solution | Solution A is clear and colourless. |
| Acidity or Alkalinity | Not more than 0.5 ml of 0.05M sulphuric acid or 0.1M sodium Hydroxide is required. |
| Arsenic | Not more than 2 ppm |
| Heavy Metals | Not more than 10 ppm |
| Chloride | Not more than 100 ppm |
| Oxalate | Not more than 300 ppm (calculated as anhydrous oxalic acid). |
| Sulphate | Not more than 150 ppm |
| Tartrate | No crystalline precipitate is formed. |
| Readily Carbonisable Substances | The solution is not more intensely coloured than reference Solution YS2 or GYS2. |
| Water | 11.0 to 13.0 per cent, determined on 0.3 g. |
| Assay | 99.0 % to 101.0 % (On Anhydrous Basis) |
JP

| Tests | Specifications |
|---|---|
| Description | White, granular crystals or white crystalline powder; odourless; Slightly deliquescent in moist air. |
| Solubility | Freely soluble in water; practically insoluble in ethanol (95%) and in ether. |
| Identification A. Citrates | 1. A dense, white precipitate is formed. 2. A yellow, crystalline precipitate is formed. |
| Identification B. Sodium | 1. A white precipitate soluble in 6M acetic acid. |
| Appearance of Solution | Solution A is clear and colourless. |
| Acidity or Alkalinity | Not more than 0.5 ml of 0.05M sulphuric acid or 0.1M sodium Hydroxide is required. |
| Arsenic | Not more than 2 ppm |
| Heavy Metals | Not more than 10 ppm |
| Chloride | Not more than 100 ppm |
| Oxalate | Not more than 300 ppm (calculated as anhydrous oxalic acid). |
| Sulphate | Not more than 150 ppm |
| Tartrate | No crystalline precipitate is formed. |
| Readily Carbonisable Substances | The solution is not more intensely coloured than reference Solution YS2 or GYS2. |
| Water | 11.0 to 13.0 per cent, determined on 0.3 g. |
| Assay | 99.0 % to 101.0 % (On Anhydrous Basis) |
KP

| Tests | Specifications |
|---|---|
| Description | White, granular crystals or white crystalline powder; odourless; Slightly deliquescent in moist air. |
| Solubility | Freely soluble in water; practically insoluble in ethanol (95%) and in ether. |
| Identification A. Citrates | 1. A dense, white precipitate is formed. 2. A yellow, crystalline precipitate is formed. |
| Identification B. Sodium | 1. A white precipitate soluble in 6M acetic acid. |
| Appearance of Solution | Solution A is clear and colourless. |
| Acidity or Alkalinity | Not more than 0.5 ml of 0.05M sulphuric acid or 0.1M sodium Hydroxide is required. |
| Arsenic | Not more than 2 ppm |
| Heavy Metals | Not more than 10 ppm |
| Chloride | Not more than 100 ppm |
| Oxalate | Not more than 300 ppm (calculated as anhydrous oxalic acid). |
| Sulphate | Not more than 150 ppm |
| Tartrate | No crystalline precipitate is formed. |
| Readily Carbonisable Substances | The solution is not more intensely coloured than reference Solution YS2 or GYS2. |
| Water | 11.0 to 13.0 per cent, determined on 0.3 g. |
| Assay | 99.0 % to 101.0 % (On Anhydrous Basis) |
To Know more
about our products
Daffodil Pharmachem, Asia’s largest WHO-GMP certified citrate manufacturer, produces over 80 metric tonnes daily, serving trusted global brands across food, pharma, agriculture, and healthcare. Our ingredients quietly enhance products that millions rely on every day—because, for us, chemistry is more than science; it’s our way of Adding Life.
Quick Contact
Daffodil Pharmachem Pvt. Ltd.
A-2304, Privilon, 23rd Floor
B/H Iscon Temple, Ambli-Bopal Road,
S.G. Highway, Ahmedabad-380054
Gujarat, India
©2024 Daffodil pharmachem. All rights reserved

