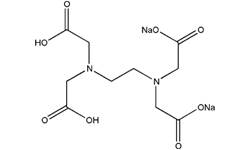
Di Sodium Edetate
Description
Disodium Edetate is used as a Pharmaceutical aid, a sequestering agent and a chelating agent in Cosmetic Industry & detergent Industry.
Applications and Effects
It is used for bringing more lather in the detergents.
Specifications
| Tests | Specifications |
|---|---|
| Description | White crystalline powder. |
| Solubility | Soluble in water. |
| Identification | (A) By Infrared Absorption. Undried. |
| (B) The red color is discharged, leaving a yellowish solution. | |
| (C) A dense precipitate is formed. | |
| pH | Between 4.0 and 6.0 in a solution (1 in 20). |
| Loss on drying | 8.7% to 11.4%, Dry it at 150° C for 6 hours. |
| Calcium | No precipitate is formed. |
| Heavy Metals | Not more than 0.005% (Method II). |
| Limit of nitrilotriacetic acid | Not more than 0.1%. |
| Assay | 99.0 % to 101.0 %, on dried basis |
| Tests | Specifications |
|---|---|
| Appearance | White or almost whiter crystalline powder. |
| Solubility | Soluble in water; Practically insoluble in ethanol (96%). |
| Identification | First identification A, B, D. Second Identification B,C, D. |
| (A)The infrared absorption spectrum of the sample is con Concordant with the spectrum obtained from Disodium Edetate working standard. | |
| (B)No precipitate is formed. | |
| (C)No precipitate is formed. | |
| (D)Gives the reactions of Sodium (A)A dense, white precipitate is formed. (B)No precipitate is formed. | |
| Appearance of solution | Solution S is clear and Colourless. |
| pH | 4.0 to 5.5 for solution S |
| Impurities A | Not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (0.1 %). |
| Iron | Not more than 80 ppm |
| Heavy Metals | Not more than 20 ppm |
| Assay | 98.5 % to 101.0 % |
| Tests | Specifications |
|---|---|
| Description | A white, crystalline powder; odourless |
| Solubility | Soluble in water; sparingly soluble in ethanol (95%), Practically insoluble in chloroform and in ether. |
| Identification | (A) Determine by infrared absorption spectrophotometry. Compare the spectrum with that obtained with disodium edetate RS. |
| (B) No precipitate is produced. | |
| (C) No precipitate is produced. | |
| (D) Gives the reactions of sodium salts (A) A dense, white precipitate is formed. (B) A yellow, crystalline precipitate is formed. | |
| Appearance of solution | 5.0 per cent w/v solution in carbon dioxide-free water is clear and colourless. |
| pH | 4.0 to 5.5 determined in 5.0 per cent w/v solution. |
| Impurity A | Not more than 0.1 per cent. |
| Heavy Metals | Not more than 20 ppm |
| Iron | Not more than 80 ppm |
| Assay | 98.5 % to 101.0 % |
| Tests | Specifications |
|---|---|
| Description | A white, crystalline powder; odourless |
| Solubility | Soluble in water; sparingly soluble in ethanol (95%), Practically insoluble in chloroform and in ether. |
| Identification | (A) Determine by infrared absorption spectrophotometry. Compare the spectrum with that obtained with disodium edetate RS. |
| (B) No precipitate is produced. | |
| (C) No precipitate is produced. | |
| (D) Gives the reactions of sodium salts (A) A dense, white precipitate is formed. (B) A yellow, crystalline precipitate is formed. | |
| Appearance of solution | 5.0 per cent w/v solution in carbon dioxide-free water is clear and colourless. |
| pH | 4.0 to 5.5 determined in 5.0 per cent w/v solution. |
| Impurity A | Not more than 0.1 per cent. |
| Heavy Metals | Not more than 20 ppm |
| Iron | Not more than 80 ppm |
| Assay | 98.5 % to 101.0 % |
