Potassium Citrate
Description
Potassium Citrate is used as a pharmaceutical aid in veterinary practice. It is also used in many pharmacopoeia and other preparations like potassium hydroxide solution, Cresol solution, Soap solution etc. It works as a buffer for juices like real, R-cola etc.
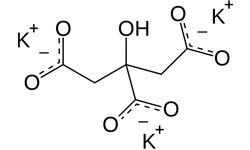
Applications and Effects
Potassium Citrate is mainly used for making solutions in the Pharmaceutical Industry which helps to make parenteral nutrition (the liquid used for making the Injections)
Specifications
| Tests | Specifications |
|---|---|
| Description | Transparent crystals or white granular powder; odorless saline taste, and is deliquescent when exposed to moist air. |
| Solubility | Freely soluble in water. Almost insoluble in alcohol. |
| Identification | A) A solution (1 in 20) responds to the test for Potassium and for citrate. Potassium: A White crystalline precipitate that is soluble in 6N ammonium hydroxide. Citrate: A light red color is produced. |
| Alkalinity | No pink color is produced by 1 drop of phenolphthalein TS. |
| Loss on drying | Between 3.0% and 6.0%. Dry it at 180°C for 4 hours. |
| Tartrate | No precipitate is formed. |
| Heavy Metals | Not more than 0.001%. |
| Assay | 99.0 % to 100.5 % (On dried basis) |
| Tests | Specifications |
|---|---|
| Appearance | White or almost white, granular powder or transparent crystals, hygroscopic. |
| Solubility | Very soluble in water, practically insoluble in ethanol (96%). |
| Identification A. Citrates | 1. Violet colour, turning to violet-blue is produced. 2. A white precipitate is produced which is soluble in 6M acetic acid. |
| Identification B. Potassium | 1. A yellow or orange-yellow precipitate is produced immediately. |
| Appearance of Solution | Solution S is clear and colourless. |
| Acidity or Alkalinity | Not more than 0.2 ml of 0.1M hydrochloric acid or 0.1M Sodium Hydroxide is required to change the colour of the solution. |
| Readily Carbonisable Substances | The solution is not more intensely coloured than reference solution Y2 or GY2. |
| Chloride | Not more than 50 ppm |
| Oxalate | Not more than 300 ppm (calculated as anhydrous oxalic acid). |
| Sulphate | Not more than 150 ppm |
| Heavy Metals | Not more than 10 ppm |
| Sodium | Not more than 0. 3 % |
| Water | 4.0 to 7.0 per cent, determined on 0.250 g. |
| Assay | 99.0 % to 101.0 % (On Anhydrous Basis) |
| Tests | Specifications |
|---|---|
| Description | White granular crystals or a white crystalline powder; odourless; hygroscopic. |
| Solubility | Very soluble in water; soluble in glycerin; practically insoluble in ethanol (95%). |
| Identification A. Potassium Salts | 1. A yellow or orange-yellow precipitate is produced. 2. A white crystalline precipitate is produced. 3. A yellow crystalline precipitate is produced which on Ignition leaves a residue of kcl and platinum. |
| Identification B. Citrates | 1. A white precipitate soluble in 6M acetic acid is produced. 2. A violet colour which turns violet-blue is produced. |
| Appearance of Solution | Solution A is clear and colourless. |
| Acidity or Alkalinity | Not more than 0.2 ml of 0.1M hydrochloric acid or 0.1M sodium Hydroxideis required to change the colour of the solution. |
| Arsenic | Not more than 2 ppm |
| Heavy Metals | Not more than 10 ppm |
| Sodium | Not more than 0.3 % |
| Chloride | Not more than 100 ppm |
| Oxalate | Not more than 300 ppm (calculated as anhydrous oxalic acid). |
| Sulphate | Not more than 150 ppm |
| Readily Carbonisable Substances | The solution is not more intensely coloured than reference solution YS2 or GYS2 |
| Water | 4.0 to 7.0 per cent, determined on 0.5 g. |
| Assay | 99.0 % to 101.0 % (On Anhydrous Basis) |
